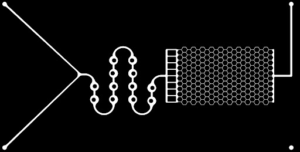
How do you know that the oil doesn’t rise above the water in the channels, or pores, of your microfluidic chips as a result of the density difference between the fluids?
This is a question that we often hear when talking at events or chatting with clients. In short, the answer is no, the oil doesn’t rise above the water, but let’s explore why.
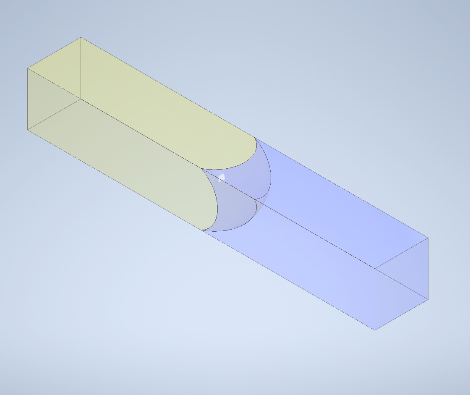
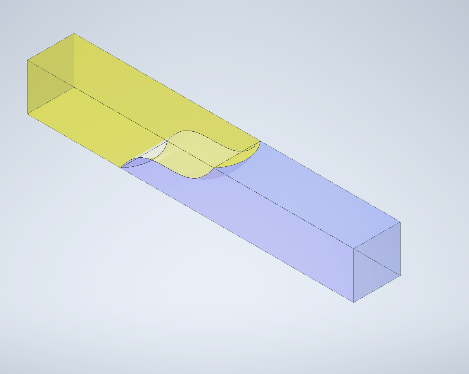
During the Interface Flowback test, our primary measurement is done through the analysis of images of our microfluidic systems. This measurement depends on the assumption that the fluid interfaces are effectively vertical.
The Experiment:
Diving deeper, an experiment was performed to evaluate the way fluids segregate in channels. We filled different size capillaries with half oil and half water and imaged the interface between them from a side view. Below is the experimental setup:
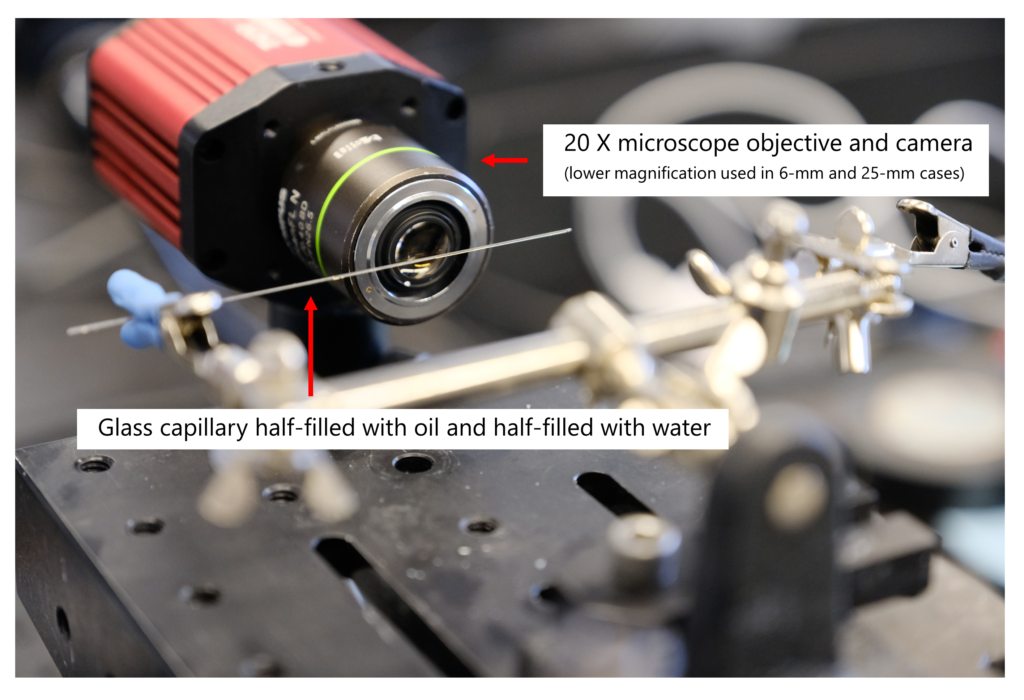
Although we run our tests using a microfluidic analogue, we used fluid-filled glass capillaries in this example to obtain the side view of the fluid interface. This setup allowed us to visually see if gravity would cause one fluid to rise above the other.
The Results:
After running the experiment, the test results in Figure 4 show that at diameters of up to 6 mm there is no gravity-wise segregation of fluids, proving that the oil-water interface is unaffected by gravity. That is, the interfacial forces dominated the gravity forces. However, in a 25-mm glass tube, the gravity effect is substantial, and there is clear separation of the oil and water. Internally at Interface, all of our tests are run in much smaller diameter channels (our tests are run in sub-0.02 mm channels), thus we know that the oil and water do not layer due to gravity.
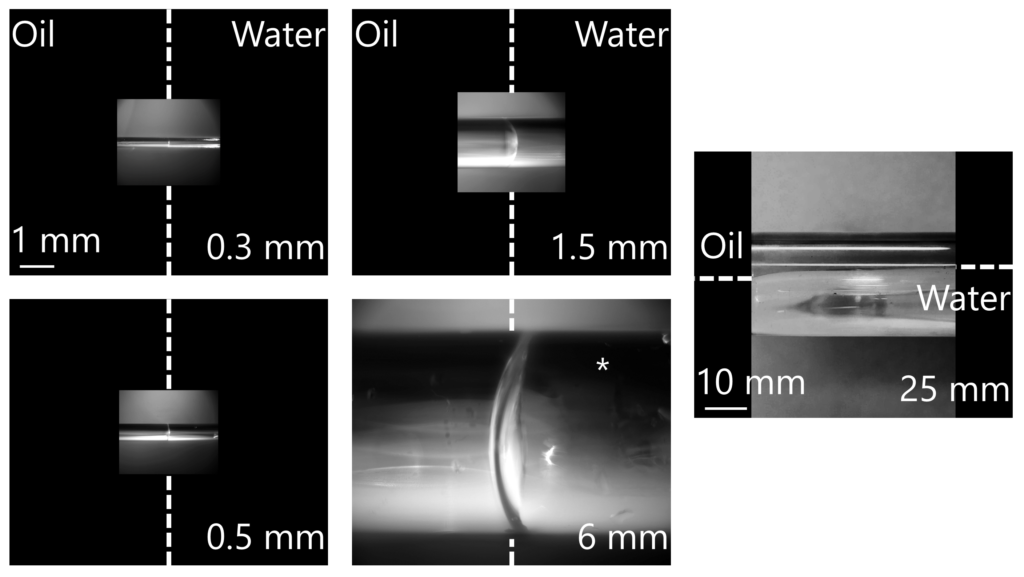
This balance of interfacial and gravity forces is well-established in the literature. Analytically, this balance is reflected in a dimensionless number from fluid dynamics called the Bond Number, Bo – also called the Eötvös Number, Eo, and no relation to 007. The Bond Number is a ratio between the gravitational forces and surface tension forces in a system. If the Bond Number is much higher than 1, then gravity dominates, and the less dense fluid will rise above the denser fluid. In such a case, the interface between the fluids would be parallel to the long channel length, as observed in the 25 mm experiment imaged above.
If the Bond Number is much smaller than 1, then surface tension will dominate, and the two fluids will remain separated with a vertical interface between the fluids – without one phase riding above the other phase.
The equation for the Bond Number is shown below:
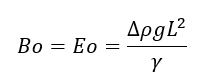
where,
Δρ is the difference in density between the two fluids (for Interface Fluidics this is usually water or brine and oil) [kg/m3]
g is gravitational acceleration [m/s2]
L is the characteristic length (in our case this is the hydraulic diameter of the microchannel) [m]
γ is the surface tension (in our case the interfacial tension is between water or brine and oil) [N/m]
In the typical case of oil and water in a capillary at room temperature with an interfacial tension of 35 nN/m, the Bond Numbers for different channel diameters are provided in Table 1.
Table 1: Bond Number of water/oil system (IFT 35 mN/m)
| Capillary Diameter | 0.02 mm | 0.3 mm | 0.5 mm | 1.5 mm | 6 mm | 25 mm |
| Bond Number | 2.4 x 10-5 | 0.0053 | 0.015
| 0.13 | 2.1 | 37 |
The addition of a surfactant, or other chemical additives, can reduce the interfacial tension between oil and water – this increases the Bond Number. Interfacial tension in enhanced oil recovery applications can be as low as 0.001 mN/m, but is more typically in the range of 0.1 to 10 mN/m. In the case of surfactant flowing through a 20-micron microchannel – which is typical for Interface’s applications – the Bond Number remains well below 1 and interfacial forces continue to dominate gravity forces (Table 2).
Table 2: Bond Number of water-oil system with surfactant (IFT 0.1 mN/m)
| Capillary Diameter | 0.02 mm | 0.3 mm | 0.5 mm | 1.5 mm | 6 mm | 25 mm |
| Bond Number | 0.0082 | 1.8 | 5.2
| 46 | 740 | 13000 |
Final Thoughts:
The discussion above considers a static system. We have described how the shape of the interface can be defined when there is no flow. In a dynamic system, other effects including inertia can influence the shape of the interface. We’ll be showcasing another experiment that highlights these interactions in a flowing system. For another interesting blog post that discusses the impact of length-scale on fluid behaviour, check out The Peclet Number and How It Describes Fluid Transport
Questions? We’d love to hear from you and understand how our testing and analysis could provide you with the data needed to make strategic completions decisions while evaluating a range of down-hole chemicals.
By: Tom de Haas, Scott Pierobon, Victor Chikhachev, and David Sinton